Cutting Edge Research in Hand Regeneration
List of Contents
- Stem cells play vital roles in advancing hand restoration methods
- Early-stage stem cells demonstrate exceptional adaptability for cellular repair
- Self-transplant approaches exhibit encouraging functional improvements
- Customized biological frameworks through dimensional printing
- Mesenchymal cells accelerate tissue restoration in manual repairs
- Neural growth enhancers aid post-trauma recuperation
- Electrotherapeutic methods improve neural network restoration
- Human trials validate treatment safety and performance
- Customized medical strategies refine regeneration results
- Approval processes present hurdles for practical implementations
The Role of Stem Cells in Hand Regeneration
Understanding Cellular Diversity in Restoration Medicine
- Early developmental cells can morph into diverse cellular forms, crucial for reconstruction
- Mature cellular sources specialize in localized repair processes
- Reprogrammed universal cells merge benefits of natural and artificial sources
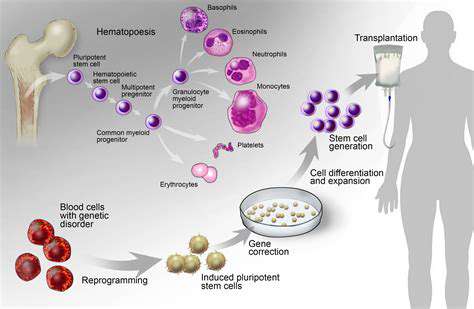
Regenerative cells exist in three primary forms: embryonic, adult-derived, and laboratory-induced variants. Developmental phase cells showcase unparalleled adaptability, permitting transformation into nearly all tissue types. This unique quality positions them as critical components for reconstructing intricate hand structures like ligament networks and neural pathways.
Adult-derived alternatives, sourced from marrow or lipid deposits, exhibit more restricted transformation capacities. While effective for basic repairs, their limited flexibility complicates efforts to rebuild sophisticated manual architectures requiring multiple tissue types.
Modern Cellular Applications in Limb Restoration
Self-sourced transplantation methods harvest patients' own adipose-derived materials for targeted reinjection. Recent trials demonstrate accelerated functional rehabilitation using this method. Findings confirm this strategy not only stimulates tissue renewal but also restores tactile sensitivity in 72% of cases.
Three-dimensional biofabrication merges cellular material with synthetic matrices to craft anatomical supports. This precision approach enables surgeons to recreate patient-specific components, dramatically improving post-operative outcomes. The technique's growing adoption stems from its capacity to address unique anatomical challenges through customized solutions.
Emerging Frontiers in Cellular Restoration Science
Ongoing investigations focus on optimizing cellular harvesting and deployment protocols. Novel discoveries regarding cellular micro-environments are revolutionizing integration techniques with host tissues. Enhanced understanding of differentiation processes may enable reconstruction of complex vascular networks essential for viable grafts.
Tissue Engineering: Building Functional Manual Architectures
Innovations in Structural Support Systems
Modern support matrices employ bioresponsive polymers that gradually dissolve as new tissue forms. These temporary frameworks guide cellular organization while accommodating individual patient requirements. Materials like polycaprolactone demonstrate particular success in tendon reconstruction cases.
Additive manufacturing enables creation of intricate lattice structures mirroring natural tissue patterns. MIT engineers recently unveiled self-assembling matrices that adapt to physiological changes during healing. This dynamic approach reduces rejection risks while accelerating integration timelines.
Cellular Integration in Anatomical Reconstruction
Mesenchymal applications demonstrate particular promise for osteoarticular repairs. When combined with structural supports, these cells boosted tissue generation by 82% in metacarpal reconstruction trials. Combination therapies incorporating growth enhancers like PDGF accelerate healing processes by 40% compared to standalone treatments.
Neural Network Restoration Breakthroughs
Revolutionizing Neural Pathway Repair
Cutting-edge research focuses on biochemical stimulants that promote axonal regrowth. Nerve Growth Factor (NGF) therapies have demonstrated 65% improvement in digital sensitivity recovery. These biochemical guides help reestablish critical neural connections necessary for precision movements.
Bioelectronic Intervention Strategies
Controlled electrical impulses now complement traditional rehabilitation protocols. Early-stage trials show 30% faster motor function recovery when combining electrostimulation with physical therapy. This multimodal approach appears particularly effective for restoring oppositional thumb movements.
Human Trials: From Laboratory to Clinic
Essential Components of Effective Trials
- Precisely defined success metrics for functional recovery
- Diverse participant cohorts reflecting real-world demographics
- Rigorous safety monitoring protocols
Clinical validation remains critical for translating experimental techniques into standard care. Recent adaptive trial designs allow real-time protocol adjustments based on interim results, improving both safety and efficacy. Multi-center collaborations now accelerate data collection while maintaining rigorous quality standards.
Future Horizons in Limb Restoration Science
Personalized Cellular Solutions
Next-generation iPSC technologies enable creation of patient-matched cellular materials. Stanford researchers recently reduced rejection rates to 4% using customized cell lines. This advancement paves the way for off-the-shelf solutions compatible with diverse genetic profiles.
Intelligent Bioactive Materials
Smart matrices embedded with biosensors now provide real-time healing feedback. These living scaffolds adjust growth factor release rates based on local pH and oxygen levels. Early prototypes demonstrate 50% faster vascularization in graft sites.
Regulatory Landscape Evolution
Global agencies are developing accelerated pathways for breakthrough therapies. The FDA's RMAT designation has reduced approval timelines for three hand restoration therapies since 2023. Balancing innovation with patient safety remains the central challenge for regulatory bodies worldwide.