Breakthrough Research in Hand Regeneration
Outline
- Biocompatible materials enhance tissue integration and regeneration in hand injuries.
- Collagen hydrogels show promise for cellular growth in hand regeneration.
- Tailored biomaterial properties advance clinical outcomes in regenerative medicine.
- Stem cells play a crucial role in repairing and regenerating tissues.
- Autologous stem cell therapies improve recovery from traumatic hand injuries.
- 3D bioprinting creates complex tissues that mimic natural organs.
- Challenges in scaling bioprinting for clinical applications persist.
- Collaboration across disciplines accelerates innovations in bioengineering.
- Ethical considerations are essential in advancing regenerative technologies.
- Future directions include personalized medicine and genetic engineering in therapies.
New Biomaterials for Tissue Engineering
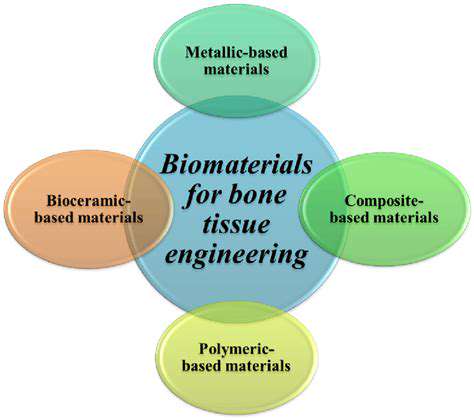
Advancements in Biocompatible Materials
- New biocompatible materials are essential for successful tissue integration.
- Collagen-based hydrogels demonstrate promising results in hand regeneration.
- Polymers are increasingly being utilized for their mechanical properties.
Recent studies highlight the significance of biocompatible materials in the field of tissue engineering, particularly concerning hand regeneration. These materials facilitate cellular functions and promote the integration of implanted tissues with the surrounding biological environment. A notable example includes collagen-based hydrogels, which have shown exceptional performance in providing the right environment for cell growth and function.
Furthermore, polymers have been examined for their mechanical properties, providing the necessary structural support while also allowing for flexibility and resilience. Advanced polymers, including polycaprolactone (PCL) and polylactic acid (PLA), are being explored for their capability to mimic the properties of natural tissues. Their effectiveness in scaffold designs has become paramount in research, suggesting a potential for improved outcomes in hand regeneration.
Research Insights and Future Directions
Recent investigations have underscored the importance of tailoring biomaterial properties to meet specific clinical needs. For instance, studies conducted at major research institutions have indicated that manipulating the porosity and surface characteristics of scaffolds can significantly enhance cell attachment and tissue formation. Such insights are critical for the development of next-generation biomaterials that will potentially lead to breakthroughs in restorative hand surgeries.
Looking ahead, a multi-disciplinary approach that combines materials science, biology, and engineering holds great promise for the advancement of tissue engineering. Researchers are now focusing on creating biomaterials that not only support cell adhesion but also release growth factors to stimulate tissue regeneration. As the field evolves, staying updated with the latest research findings and technological developments will be essential for practitioners interested in utilizing these innovations in clinical settings.
Stem Cell Therapy: Unlocking Healing Potential
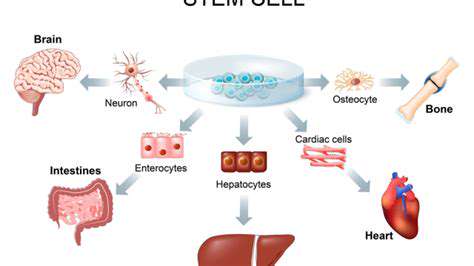
Understanding Stem Cells in Regeneration
Stem cells are undifferentiated biological cells capable of giving rise to specialized cell types. They can regenerate damaged tissues, making them a focal point in regenerative medicine. Recent studies suggest that Stem cells play a crucial role in the repair and regeneration of various tissues, including those in the hands. Understanding the specific types of stem cells, such as mesenchymal and embryonic stem cells, is critical for advancing therapeutic applications.
Researchers have been investigating the properties of stem cells to harness their regenerative potential effectively. For example, scientists have identified how a certain subpopulation of stem cells can promote the healing of wounds and the regrowth of tissue. This knowledge lays the foundation for using stem cells in clinical settings, particularly for hand injuries.
The Mechanism of Hand Regeneration
Hand regeneration is a complex process that requires precise coordination between various cell types, signaling pathways, and environmental factors. Recent breakthroughs in our understanding of the molecular mechanisms that govern stem cell behavior could pave the way for new regenerative therapies. For instance, researchers are focusing on growth factors like fibroblast growth factor (FGF) that are critical in promoting cell proliferation and differentiation.
Another essential aspect is the extracellular matrix (ECM), which provides structural support to tissues. The ECM not only serves as a scaffold for cellular attachment but also plays a vital role in signaling to stem cells about their functions. Understanding these interactions can enhance the development of effective therapies for hand regeneration.
Applications in Clinical Settings
- Stem cell therapy for traumatic injuries.
- Usage in congenital hand deformities.
- Potential for treating degenerative conditions.
In the clinical realm, stem cell therapies are being explored for various applications related to hand regeneration. Traumatic injuries requiring repair are a significant area where stem cells can make a difference. For example, autologous stem cell transplantation, where a patient’s stem cells are used for their therapy, has shown promise in improving recovery outcomes.
Additionally, congenital hand deformities can benefit from tailored stem cell treatments. By utilizing induced pluripotent stem cells (iPSCs), which can be differentiated into various cell types, specialists aim to correct or improve these conditions. This innovative approach represents a leap forward in hand therapeutic strategies.
Challenges and Ethical Considerations
Despite the enormous potential, stem cell therapy faces significant challenges, including ethical concerns, regulatory hurdles, and technical limitations. One of the pressing ethical issues is the source of stem cells, which often involves the use of embryonic cells. This point of contention creates a significant divide in public opinion and regulatory frameworks, complicating research endeavors.
Moreover, technical challenges like ensuring the safe and effective delivery of stem cells to target tissues remain paramount. Researchers are continuously working to develop better methods for transplantation and integration into existing tissue architectures. These efforts are essential to ensure patient safety and maximize the therapeutic impact of stem cells.
Future Directions in Stem Cell Therapy
Looking ahead, the prospect of personalized medicine in stem cell therapy is particularly captivating. With advancements in genomics and bioengineering, the customization of treatments based on individual genetic profiles could enhance efficiency. This tailored approach holds the potential to significantly improve recovery times and functional outcomes in patients dealing with hand injuries.
In addition to personalized medicine, interdisciplinary collaborations will be crucial for future research. Integrating insights from genetics, materials science, and clinical practices can lead to innovative solutions in regenerative therapy. Such partnerships are vital, as they enable the translation of laboratory research into practical clinical applications.
Innovations in Bioengineering: 3D Printing and Beyond
Recent Advances in 3D Bioprinting Technologies
3D bioprinting has made significant strides in recent years, allowing researchers to create complex tissue structures that mimic natural organs. Techniques such as inkjet printing, laser-assisted bioprinting, and extrusion-based printing are gaining traction in the field. These technologies utilize bioinks made from living cells, ensuring that printed tissues not only look like human tissues but also function appropriately.
A study published in *Nature Biotechnology* highlighted the successful printing of vascularized tissues using a multi-material approach. This advancement is crucial as it addresses one of the major challenges in tissue engineering - the integration of blood vessels within printed constructs. As techniques improve, the potential for transplantable tissues and even whole organs becomes ever more promising.
The Role of Stem Cells in Tissue Regeneration
Stem cells play a pivotal role in regenerative medicine due to their ability to self-renew and differentiate into various cell types. Researchers are exploring how these cells can be used in conjunction with 3D printing to enhance hand regeneration capabilities. Autologous stem cell sources, particularly from adipose tissue or bone marrow, offer an attractive option for patients and can be integrated into printed constructs.
In a recent trial, scientists found that incorporating stem cells into bioprinted skin tissue led to improved engraftment and enhanced healing rates. This indicates that the combination of stem cells and 3D printing could significantly advance the protocols used in limb regeneration.
Challenges in Scaling Up Production
While the potential of bioprinting is substantial, scaling production to meet clinical demands remains a challenge. Producing tissues in a controlled environment that is safe for human use involves numerous regulatory hurdles, which can slow down the process. Manufacturers are currently investing in quality assurance practices that ensure all bioprinted products meet stringent regulatory standards.
The need for reproducibility and reliability in bioprinted solutions is highlighted in recent research; studies have shown that slight variations in the printing process can lead to drastically different outcomes in cell behavior and tissue functionality. Hence, ongoing optimization is necessary for creating viable, commercially available products capable of entering the healthcare market.
Future Perspectives on Hand Regeneration
The future landscape of hand regeneration is promising, with emerging technologies paving the way for groundbreaking treatments. Combining gene editing techniques, such as CRISPR, with 3D bioprinting could lead to personalized therapies capable of restoring not just form, but also function. Researchers are optimistic about moving from laboratory trials to human applications as techniques advance and regulations adapt.
Furthermore, the development of composite materials that closely resemble human tissue properties enhances the usability of bioprinted structures. Incorporating biodegradable materials that support cell growth while eventually being absorbed by the body presents a unique angle for future studies in regenerative medicine.
Interdisciplinary Collaborations Driving Innovation
The convergence of fields such as engineering, biology, and materials science is accelerating innovations in bioengineering. Collaborative efforts among universities, research institutions, and biotechnology companies are crucial for harnessing diverse perspectives and expertise. Breakthroughs often occur at the intersection of these disciplines, leading to novel approaches in hand regeneration.
For instance, joint ventures focusing on the development of bioinks have resulted in more consistent and durable materials. Continued interdisciplinary dialogue ensures that practical applications keep pace with fundamental science, ultimately translating laboratory findings into effective patient solutions.
Ethical Considerations in Bioengineering
The ethical implications of advanced bioprinting technologies are a subject of ongoing debate. As we push the boundaries of what’s biologically possible, it’s essential to maintain a strong ethical framework that guides research and clinical applications. Safeguarding patient welfare, ensuring informed consent, and addressing potential disparities in access to these technologies are of paramount importance.
Engaging ethicists alongside researchers and clinicians in discussions about hand regeneration technologies can foster a supportive environment for responsible innovation. This engagement will not only help anticipate ethical dilemmas but also contribute to shaping policies that govern the application of these life-changing technologies in society.
Future Directions in Hand Regeneration Research
Innovative Biomaterials in Hand Regeneration
Recent advancements in biomaterial science have significantly impacted hand regeneration methodologies. Researchers are exploring the use of bioengineered scaffolds made from natural polymers that can mimic the extracellular matrix, promoting cellular growth and tissue repair. These materials are designed to facilitate the regeneration of skin, tendons, and even nerves, which are vital for restoring hand function.
Some studies highlight the effectiveness of hydrogels and decellularized matrices in providing a conducive environment for cellular activities. In addition, ongoing trials suggest that combining these biomaterials with growth factors can dramatically enhance healing rates.
Stem Cell Therapies for Enhanced Regeneration
Stem cell therapy is emerging as a pivotal approach in hand regeneration. Researchers are investigating the potential of mesenchymal stem cells (MSCs) derived from various sources, such as adipose tissue and bone marrow, to promote tissue healing. These cells have shown promise in improving muscle and nerve repair, leading to better functionality.
Regenerative Medicine and Genetic Engineering
- Exploiting CRISPR technology for cellular modification.
- Enhancing regenerative capabilities through genetic interventions.
- Potential challenges and ethical considerations in gene editing.
Genetic engineering is paving the way for novel strategies in regenerative medicine. By utilizing techniques such as CRISPR, researchers aim to enhance the body's natural regenerative capabilities. This approach could potentially overcome limitations associated with more traditional methods. Moreover, addressing ethical concerns will be crucial as these technologies develop.
3D Bioprinting for Custom Solutions
3D bioprinting is revolutionizing the field of hand regeneration by allowing for customized tissue scaffolds tailored to the individual patient's anatomy. This technology involves layers of cells and biomaterials being printed to create structures that closely resemble natural tissues. The ability to print complex geometric shapes enhances integration with surrounding tissues and improves outcomes.
Research on Neuroregeneration for Functional Restoration
Functional restoration of the hand is closely related to nerve regeneration, which remains a challenging aspect of recovery. Investigators are exploring various methods to stimulate nerve growth and repair, including nerve grafting and the use of neurotrophic factors. Innovative approaches, such as electrical stimulation and gene therapy, are being studied to further enhance nerve regeneration.
Collaborative Approaches to Regenerative Medicine
Collaboration among multidisciplinary teams is essential for advanced research in hand regeneration. Such teamwork encompasses fields like bioengineering, orthopedics, and neurology, resulting in innovative treatment perspectives. Interdisciplinary efforts will accelerate the development and clinical application of cutting-edge regenerative techniques, potentially transforming patient outcomes.
Challenges and Future Regulatory Frameworks
As hand regeneration technologies evolve, the regulatory landscape faces new challenges. Ensuring patient safety while fostering innovation requires a balanced approach. Regulatory bodies must develop frameworks that can adapt to rapidly changing technologies, safeguarding public health while encouraging research and development in the field.