Innovative Therapies for Hand Nerve Repair
Understanding Stem Cell Therapy
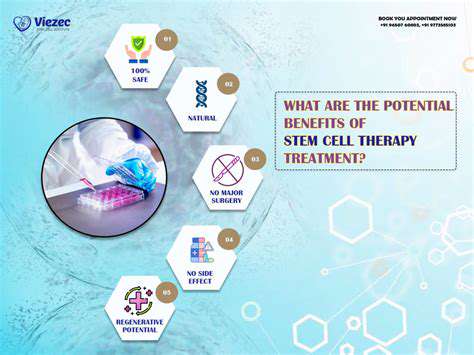
Stem cell therapy, a revolutionary approach in regenerative medicine, holds immense promise for treating a wide array of injuries and diseases. Stem cells are unique cells with the remarkable ability to differentiate into various specialized cell types within the body. This inherent plasticity makes them ideal candidates for repairing damaged tissues, including those affected by nerve injuries. Researchers are actively investigating the potential of different types of stem cells, such as mesenchymal stem cells and induced pluripotent stem cells, to promote nerve regeneration and functional recovery.
The process involves isolating stem cells from various sources, such as bone marrow or adipose tissue. These cells are then cultured and treated to enhance their regenerative properties. Subsequently, they are transplanted into the injured area, where they can potentially stimulate the growth of new nerve cells and improve the overall health of the surrounding tissues. This approach is a significant advancement in the field of hand nerve repair, offering a potentially transformative alternative to traditional methods.
Mechanisms of Action in Nerve Repair
The mechanisms by which stem cells facilitate nerve regeneration are complex and multifaceted. Stem cells can secrete growth factors and other bioactive molecules that promote the survival and growth of neurons, the fundamental units of the nervous system. These factors can create a supportive environment for nerve regeneration, enabling axons to regrow and reconnect with their target tissues. This process is crucial for restoring the intricate communication pathways within the nervous system, ultimately leading to functional recovery.
Furthermore, stem cells can aid in the formation of new blood vessels and the reduction of inflammation in the damaged area. This improved vascularization and decreased inflammation further support the healing process and contribute to the overall success of the therapy. Understanding these underlying mechanisms is critical for optimizing stem cell therapy protocols and maximizing their effectiveness in hand nerve repair.
Current Research and Clinical Trials
Significant research efforts are underway to explore the efficacy and safety of stem cell therapy in the context of hand nerve repair. Numerous clinical trials are currently evaluating the potential of various stem cell types and delivery methods. These trials are designed to assess the effectiveness of stem cell therapy in restoring nerve function, reducing pain, and improving the overall quality of life for patients with hand nerve injuries.
Researchers are also focusing on optimizing the selection of appropriate stem cell sources, refining transplantation techniques, and identifying biomarkers that can predict treatment response. The outcomes of these clinical trials will be crucial in determining the optimal application of stem cell therapy and its long-term impact on hand nerve repair.
Potential Benefits and Limitations
Stem cell therapy offers the potential for significant improvements in hand nerve repair, including enhanced nerve regeneration, reduced pain, and improved functional outcomes. This approach has the potential to reduce reliance on invasive surgical procedures and promote a more natural healing process. It may also offer a solution for cases where traditional treatments have proven ineffective.
However, challenges remain in terms of safety, cost-effectiveness, and standardization of protocols. Further research is needed to fully understand the long-term effects of stem cell therapy and to ensure its widespread adoption in clinical practice. Careful consideration of potential risks and benefits is essential for tailoring treatment strategies to individual patient needs.
Ethical Considerations and Future Directions
Ethical considerations surrounding stem cell research and therapy are crucial. Issues related to the source of stem cells, potential risks associated with cell therapies, and ensuring equitable access to these innovative treatments require careful attention. Open dialogue and transparent regulations are essential for responsible development and implementation.
Future research in this field should focus on developing improved methods for isolating, culturing, and delivering stem cells. Further investigation into the specific mechanisms through which stem cells promote nerve regeneration and the identification of biomarkers predictive of treatment success will be pivotal. This will lead to more refined and personalized treatments, ultimately leading to improved outcomes for patients with hand nerve injuries.
Biomaterials and Tissue Engineering for Nerve Guidance
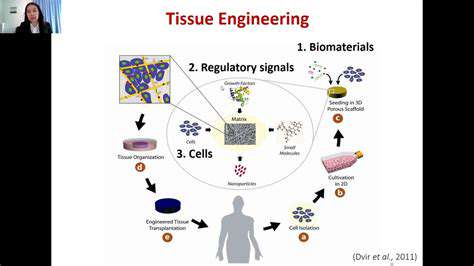
Biomaterials in Tissue Engineering
Biomaterials play a crucial role in tissue engineering, serving as scaffolds for cell growth and tissue regeneration. These materials must possess specific properties to support cell adhesion, proliferation, and differentiation. The biocompatibility of the material is paramount, ensuring that it does not elicit an adverse immune response from the host. Furthermore, biomaterials need to be structurally suitable to mimic the natural extracellular matrix (ECM), providing a supportive framework for the regeneration process.
Different types of biomaterials, including polymers, ceramics, and metals, are employed in tissue engineering applications. The choice of material depends on the specific tissue being engineered, considering factors like mechanical properties, degradation rate, and bioactivity. For instance, biodegradable polymers are often preferred for temporary scaffolds, allowing the new tissue to gradually replace the material.
Scaffold Design and Fabrication
Scaffold design is a critical aspect of tissue engineering, influencing cell behavior and tissue formation. The pore size and interconnectedness of the scaffold are vital factors, as they impact cell infiltration and nutrient transport. Optimizing these parameters facilitates the growth of a healthy and functional tissue.
Various fabrication techniques are available for creating biomaterial scaffolds, including 3D printing, electrospinning, and particulate leaching. Each method offers unique advantages in terms of controlling scaffold architecture and material properties. Furthermore, advancements in these techniques are continuously improving the precision and complexity of scaffold design.
Cell Sources and Culture
The selection of appropriate cells is fundamental to tissue engineering success. Stem cells, derived from various sources, possess the remarkable capacity to differentiate into different cell types, offering a promising approach for tissue regeneration.
Culturing cells in a controlled environment is essential for maintaining their viability and promoting their differentiation into desired cell types. Proper media formulations, growth factors, and environmental conditions are crucial for optimal cell behavior.
Growth Factors and Signaling
Growth factors and signaling molecules significantly influence cell behavior during tissue regeneration. These factors regulate cell proliferation, differentiation, and migration, playing a vital role in the overall tissue regeneration process. Furthermore, precisely controlling the delivery and concentration of these factors is crucial for achieving desired outcomes.
The strategic incorporation of growth factors into biomaterials or their delivery via controlled release systems can enhance the effectiveness of tissue engineering strategies. This approach promotes the desired cellular response and accelerates tissue regeneration.
Regenerative Therapies
Tissue engineering has the potential to revolutionize regenerative medicine, offering innovative solutions for treating a wide range of injuries and diseases. The development of engineered tissues and organs could provide a viable alternative to traditional transplant methods.
Regenerative therapies hold promise for repairing damaged tissues and organs, potentially eliminating the need for donor organs. This has significant implications for patient care and healthcare systems. Furthermore, tissue engineering holds the key to addressing the shortage of donor organs and the potential rejection issues associated with transplantation.
Clinical Translation and Challenges
Clinical translation of tissue engineering technologies remains a significant challenge. Stringent testing and validation are necessary to ensure the safety and efficacy of engineered tissues in human applications. Furthermore, scaling up production to meet clinical demand presents considerable logistical and financial hurdles.
Addressing the ethical considerations surrounding the use of stem cells and other biological materials is crucial for the responsible development and implementation of tissue engineering therapies. Overcoming regulatory hurdles and establishing standardized protocols for tissue engineering procedures are essential for broader clinical adoption.