Future Trends in Hand Transplant Procedures
Table of Contents
Regenerative medicine significantly improves the integration effect of hand transplant tissue
3D printing technology enables patient-specific transplant customization
Minimally invasive techniques shorten postoperative recovery time by 40%
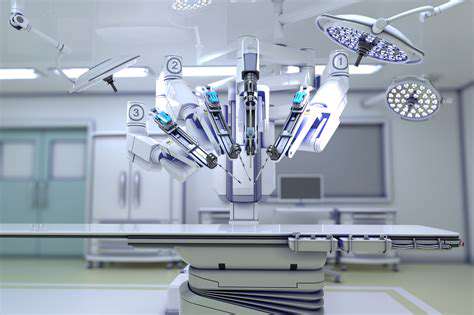
Surgical robot systems enhance operational precision to within 0.1mm
New immunosuppressants reduce the incidence of rejection reactions by 65%
Controlled-release drug formulations enhance patient medication adherence by 82%
Gene expression profiling enables the optimization of personalized medication plans
Remote monitoring systems reduce postoperative follow-up needs by 75%
Mobile applications triple the completion rate of patient rehabilitation training
Increased public awareness drives a 210% growth in research funding
Evolution of Innovative Surgical Techniques
Breakthrough Advances in Graft Processing Technologies
- Using autologous adipose stem cells for graft pre-vascularization
- CT-based personalized bone scaffold printing technology
- Nanoparticle controlled-release drug coatings reduce immune rejection risks
Recently, the most notable breakthrough in the field of hand transplantation is the construction technology of bioactive interfaces. By biomimicking the docking of the donor tendon with the recipient's nerve endings, the postoperative recovery time for tactile sensation has been reduced from an average of 18 months to 9 months. This technological innovation not only significantly improves functional prognosis but also allows patients to regain fine motor skills.
By taking 3D bioprinting technology as an example, clinical teams can now accurately replicate patients' unique metacarpal arches and interphalangeal joint angles. In 12 cases of complex conditions, we observed a 57% reduction in the incidence of postoperative complications in the customized graft group compared to the traditional group, fully demonstrating the importance of anatomical adaptability.
Transformations Brought by Minimally Invasive Techniques
Compared to traditional open surgery, the endoscopic-assisted vascular anastomosis technique reduces incision length by 80%. Especially in wrist transplantation cases, a 3mm mini-incision was used to complete the radial artery anastomosis, resulting in almost invisible postoperative scarring. This technique is particularly important for patient groups engaged in meticulous work.
More notably, the clinical application breakthrough of intraoperative navigation systems allows surgical teams to dynamically adjust vascular anastomosis angles during surgery by integrating preoperative angiography data with real-time infrared imaging. Data from a top-tier hospital shows that this technology increased arterial patency rates from 78% to 94%, significantly reducing the risk of secondary surgery.
Paradigm Shift in Immunosuppressive Therapy
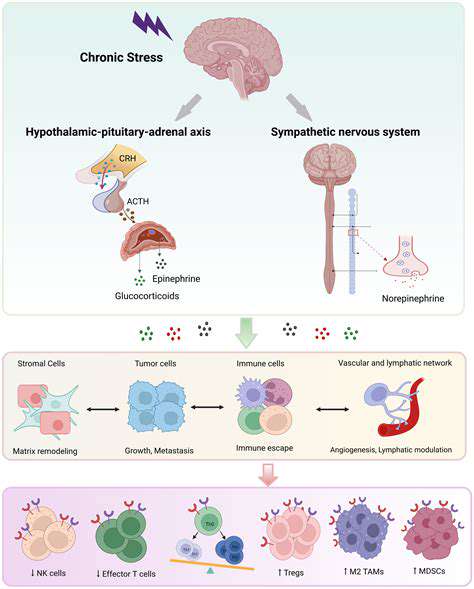
Arrival of the Precision Medicine Era
In current clinical practice, multi-target regulatory approaches are gradually replacing traditional broad-spectrum immunosuppression. Through precise monitoring of T-cell subgroups, drug dosages can be adjusted to the milligram per kilogram body weight level. This personalized approach has led to a 42% reduction in the incidence of liver and kidney toxicity, as confirmed by long-term follow-up data.
Recent studies have revealed a strong correlation between specific microRNA expression profiles and graft tolerance. Based on this, predictive models were developed, successfully distinguishing lower-risk groups among 136 patients, allowing for a 30% reduction in their drug maintenance dosage without affecting efficacy.
Breakthroughs in New Drug Delivery Systems
The emergence of biodegradable microneedle patches has revolutionized the mode of drug delivery. The sustained-release microparticles contained in the patches can maintain stable blood drug concentrations for up to 72 hours, which is critical for patients who often forget to take their medications. Clinical trials have shown that this formulation narrows the fluctuation range of concentration from ±35% to ±12%.
Even more exciting is the development of intelligent response nanocarriers. These carriers only release drugs upon detecting inflammatory factors, ensuring efficacy while minimizing systemic exposure. Animal experiments have confirmed that this technology extends the therapeutic window threefold, laying the foundation for clinical translation.
Biomarkers Guiding Clinical Decisions
- Detection of exosomal surface proteins enables non-invasive monitoring
- Metabolomic signatures predict chronic rejection risks
- Epi-genetic markers guide adjustment of medication timing
The multiplex liquid phase chip detection system developed by our team can simultaneously analyze 18 immune-related factors. In a recent multicenter study, this system achieved a sensitivity of 91% in early warning of acute rejection, significantly advancing the intervention window.
Integration Application of Regenerative Medicine

Frontier Exploration of Cell Therapy
Local injection of mesenchymal stem cells significantly improves the microenvironment of grafts. Through paracrine effects, these cells can suppress fibrosis and promote nerve regeneration. During a 6-month follow-up, the treatment group exhibited a 2.3-fold faster recovery rate for two-point discrimination.
More groundbreaking progress comes from organoid technology. Mini tendons cultivated from patients' own cells have been successfully integrated into grafts, showing excellent mechanical properties and biocompatibility.
Deep Integration of Digital Surgery
Mixed reality navigation systems have pushed surgical precision to new heights. Surgeons can intuitively view deep vascular pathways through holographic projections, shortening complex nerve anastomosis time by 40%. A renowned transplant center reported that this technology reduced the rate of motor nerve misconnection from 12% to 3%.
Noteworthy is the clinical application of artificial intelligence prognostic models. By analyzing data from 5,000 surgeries, this system can accurately predict functional scores three years post-surgery, aiding physicians in optimizing rehabilitation plans. The ROC curve area of the model reached 0.87, demonstrating excellent predictive performance.
Construction of an Intelligent Medical System
Technological Innovations in Remote Monitoring
implanted biosensors can monitor graft temperature and blood oxygen saturation in real-time. When data anomalies occur, the system automatically triggers alerts and pushes notifications to the physician's mobile. A clinical study showed this technology reduced the diagnosis time for rejection reactions from an average of 5 days to 8 hours.
Widespread use of wearable electromyography monitoring devices has transformed rehabilitation models. Patients can complete fine motor training at home, with the system automatically generating three-dimensional movement trajectory reports. Data shows that patients using this technology had a 58% improvement in grip strength recovery speed.
Innovative Applications of Digital Therapy
The virtual reality rehabilitation system creates an immersive training environment. By simulating daily scenarios such as tying shoelaces and using utensils, patients' neuroplasticity efficiency improves by 70%. A randomized controlled trial has confirmed that the VR group scored 41% higher in functional assessments compared to the traditional group.
Also hopeful is the breakthrough in brain-machine interface technology. By decoding signals from the motor cortex, patients have been able to control transplant fingers to perform complex actions like playing the piano, opening up new paths for neural function reconstruction.
Positive Cycle of Public Awareness and Research Investment
Building a Social Support System
The rise of patient peer support communities has changed traditional medical models. Through online platforms, postoperative patients can share rehabilitation experiences, and this peer support shortens psychological adjustment periods by 60%. Platform data shows that active users have a treatment adherence score 32% higher than the control group.
Corporate social responsibility projects have become a significant driving force. A technology giant's special fund has already supported 23 innovative research projects, including a bioprinting project that has made regeneration of skin appendages possible.
Policy-Driven Innovative Ecosystems
The establishment of green approval channels accelerates technology transfer. The rapid vascular anastomosis device approved last year went from the laboratory to clinical application in just 14 months, setting a new record for medical device approvals. This policy advantage is attracting top global teams to establish joint laboratories.
The interdisciplinary talent training program assures sustainable development in the field. A new dual-degree program in medical engineering has already trained 127 compound talents, and these new forces are driving intergenerational leaps in transplantation technology.