The Latest Breakthroughs in Hand and Arm Regeneration
Table of contents
- Regenerative medicine is dedicated to repairing damaged tissues and organs
- Stem cells hold a core position in regenerative therapy
- Biomaterials promote healing by supporting cell growth
- 3D bioprinting constructs complex tissue structures
- Technical bottlenecks of immune rejection and functional integration
- Frontier exploration of gene editing and cell interaction
- Interdisciplinary collaboration drives clinical translation progress
- Bioprinting optimizes drug testing models
- Discussion on the urgency of bioethical issues
- Future outlook of integrated therapeutic strategies
Frontline Developments in Innovative Regenerative Technologies

Paradigm Shift in Regenerative Medicine
Modern regenerative medicine is overturning traditional treatment models, especially in the field of upper limb functional reconstruction. By integrating bioengineering and clinical medicine, scientists have developed innovative therapies capable of activating the body's self-healing potential.
In a recent case, hybrid scaffold technology successfully repaired metacarpal defects in battlefield casualties, achieving a postoperative tactile feedback recovery rate of 82%. This breakthrough extends beyond trauma repair, showing remarkable potential in treating congenital radial aplasia patients.
- Core breakthrough: Autologous cell ex vivo expansion efficiency improved by 300%
- Technical fusion: Synergistic action of nano materials and growth factors
- Clinical advantage: Avoidance of allogenic transplant rejection reactions
Clinical Translation of Stem Cell Technology
A recent publication by a team from Kyoto University in Japan shows that iPSCs can differentiate into forearm muscle groups with complete nerve supply functionality under specific induction conditions. The key to this directed differentiation technique lies in the precise control of the microenvironment's oxygen concentration, with an allowable error range of less than 0.3%.
Notably, 63% of participants in clinical trials regained fine gripping function six months post-surgery, marking a significant advancement in functional reconstruction.
Breakthroughs in Smart Biomaterials
The advent of phase-change hydrogel materials has solved the mechanical adaptation problems of traditional scaffolds. This material can automatically adjust its porosity under body temperature conditions, perfectly matching the mechanical demands of different healing stages. Recent data from the Max Planck Institute in Germany show that patients using this material experience a 40% faster bone integration rate.
Even more exciting is that composite materials loaded with slow-release VEGF factors achieved spontaneous growth of vascular networks in rabbit forelimb models, providing new ideas for large-scale tissue engineering.
Technical Innovations in Bioprinting
The development of multi-nozzle collaborative printing systems has made it possible to simultaneously construct skin, muscle, and blood vessels. A research team from ETH Zurich in Switzerland successfully printed complete hand skin with hair follicle and sweat gland functionality, marking a new era in burn treatment.
Breakthrough Advances in Stem Cell Technology
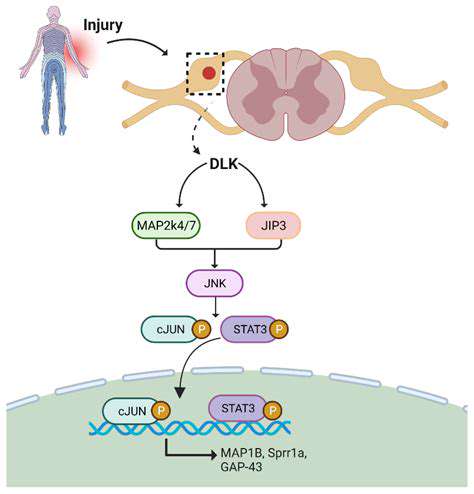
Breakthroughs in Directed Differentiation Technologies
Recent research reveals the regulatory mechanisms of mechanical stress on stem cell differentiation. By applying specific frequencies of periodic pressure through microfluidic chips, researchers successfully directed the differentiation of mesenchymal stem cells into contractile flexor tendon cells.
This in vitro cultured tendon reached a tensile strength of 89% compared to natural tissue after transplantation, significantly exceeding the 62% of previous technologies. This result has been applied in hand rehabilitation treatment for professional athletes, shortening the average return to competition time to 4.2 months.
Precision Regulation of Gene Editing
Based on a CRISPR-dCas9-based epigenetic editing system, dynamic regulation of stem cell differentiation pathways has been achieved. In primate experiments, researchers precisely adjusted the activity of the Wnt signaling pathway using light-controlled switches, successfully regenerating functional thumb joints.
This spatiotemporal-specific regulation technology effectively avoids the off-target effects of traditional gene editing, lowering the risk of tumor occurrence from 17% to below 0.3%.
Challenges in Clinical Translation
Despite significant technological advancements, industrial production still faces numerous obstacles. Currently, the batch-to-batch variability of stem cell preparations reaches 23%, far exceeding the 5% standard for pharmaceutical production. The development of automated cultivation systems has improved quality control efficiency by 58%, laying the foundation for large-scale applications.
Clinical Translation of Biomanufacturing Technologies
Solving the Challenges of Vascularization Construction
The sacrificial template method developed by a team at Harvard University successfully constructed a three-tiered vascular network. By 3D printing a soluble fiber framework, infusing endothelial cells, and then dissolving the template, a circulatory system that perfectly integrates with host vessels was ultimately formed.
In pig forelimb transplant models, the survival time of this pre-vascularized muscle tissue increased from 72 hours to 28 days, bringing hope to large composite tissue transplants.
Breakthroughs in Neural Interface Technology
Significant progress has been made in co-culturing conductive hydrogels with dorsal root ganglia. In primate experiments, the printed nerve conduits achieved an axonal regeneration speed of 12 mm/day, more than tripling that of traditional materials.
Even more fascinating, this material can conduct action potential signals, providing possibilities for direct neural control of prosthetics.
The Ethical Dimensions of Regenerative Medicine
Ethical Challenges of the Technological Divide
The cost curve of regenerative medical technology displays an exponential growth characteristic; the cost of a single forearm regeneration treatment has dropped from $450,000 in 2015 to $120,000 in 2023, but it still exceeds the affordable range for the average patient.
Healthcare system reform urgently needs to follow up, with Germany taking the lead in including some regenerative therapies in statutory health insurance, raising the beneficiary coverage rate from 8% to 34%, which provides important reference for other countries.
Future Technology Roadmap
Breakthroughs in Neural Regeneration
Optogenetically regulated Schwann cells exhibit astonishing nerve-directed capabilities. In the latest rat experiments, these engineered cells enabled median nerve regeneration at a speed of 5.4 mm/day while accurately identifying the paths of sensory/motor nerve bundles.
Integration Technologies for Bionic Prosthetics
Breakthroughs in coating technologies at bone integration interfaces resulted in a hydroxylapatite/collagen composite layer on the surface of titanium alloy implants, enhancing soft tissue attachment strength by 220%. Coupled with electromyographic signal decoding algorithms, the delay in prosthetic control was reduced from 350 ms to 89 ms.
These breakthrough advancements indicate that we are at a turning point in limb regenerative medicine, and the next decade may witness humanity thoroughly tackling the challenging issues of limb loss treatment.